HIGHER TIER
PAPER 2
TIME 2 hours 15 minutes
INSTRUCTIONS TO CANDIDATES
Write your name, Centre number and candidate number in the spaces at the top of this page.
Answer ALL questions.
Write your answers in the spaces provided on the question paper.
INFORMATION FOR CANDIDATES
The number of marks is given in brackets [ ] at the end of each question or part question.
The marks allocated and the spaces provided for your answers are a good indication of the length of answer required.
A copy of the Periodic Table is printed at the end of the question paper.
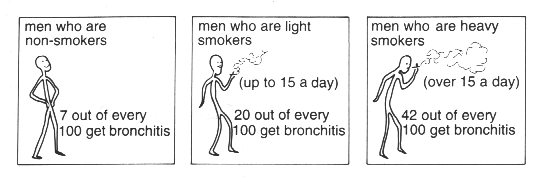
1 Bronchitis is an infection of the bronchial tubes which lead to the lungs.
(a)

What general pattern is shown by this information?
......................................................................................................................................................
.............................................................................................................................................. [1]
(b) The diagrams show the effects of cigarette smoke on small hairs called cilia in the bronchial tubes.
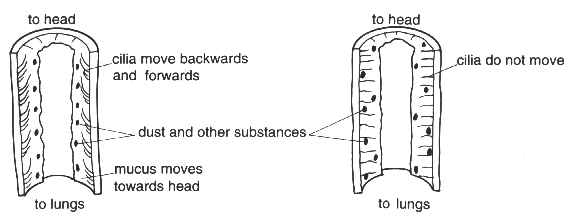
(i) What effect does cigarette smoke have on the movement of mucus?
......................................................................................................................................................
.............................................................................................................................................. [1]
(ii) Suggest why smokers tend to cough more than non-smokers.
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [2]
2 (a) Information on the average amount of radiation received by a person in a year is shown in the table.
source amount/arbitary units
natural background 125.0
medical sources 55.2
occupational hazards 1.7
other radiation 2.0
(i) Give one cause of natural background radiation.
......................................................................................................................................................
.............................................................................................................................................. [1]
(ii) Explain why the dose of X-rays you receive should be measured.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [3]
(b) (i) When radon gas is kept in a sealed container for a long time small amounts of helium appear. Explain what is happening to the radon gas.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [2]
(ii) Sketch a graph on the axes below to show how the count rate of a radioactive substance will change with time.
[1]
(iii) At 9 am a radioactive substance has a count rate of 400 counts per minute (cpm). At 10 am the count rate is 200cpm. What will it be at 12 noon?
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [2]
(c) (i) The diagram represents the structure of a helium nucleus.
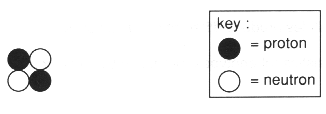
Add information to the diagram so that it represents a helium atom.[2]
(ii) Use the information in the diagram to help you explain the difference between mass number and atomic number.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [2]
3 Red-green colour blindness is a sex-linked inherited characteristic. The allele (gene) n for colour-blindness is recessive to the allele N for normal vision. The allele n is carried only on the X chromosome.
Different combinations of alleles produce different characteristics.
(a) Describe the characteristics (phenotypes) produced by these combinations.
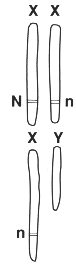 [4]
[4]
(b) In a family, Arthur could not understand why he was red-green colour blind when his brother Colin and his parents had normal vision. Use the symbols shown above to explain how this was possible.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [4]
4 Halogens are useful because they are all reactive elements.
(a) Chlorine atoms each have 17 electrons.
In the space below draw a diagram showing the arrangement of the electrons in a chlorine atom.
[3]
(b) Find fluorine in the Periodic Table at the back of this booklet.
State one similarity and one difference in the electronic structure of fluorine compared with that of chlorine.
similarity..................................................................................................................................
difference.......................................................................................................................... [2]
(c) Use your knowledge of electronic structures to explain why all the halogens are reactive.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [3]
(d) The common halogens are bromine, chlorine and iodine.
Which one is the most reactive?
.............................................................................................................................................. [1]
5 A dynamo is often used to provide electricity for bicycle lamps.
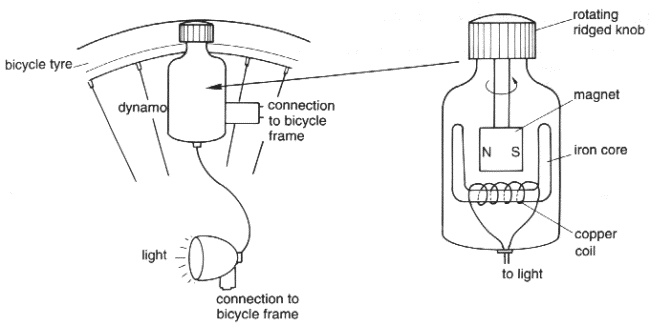
(a) Explain how an electric current is produced by the dynamo.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [4]
(b) A student used a dynamo to light a lamp.
Some current and voltage readings were taken.
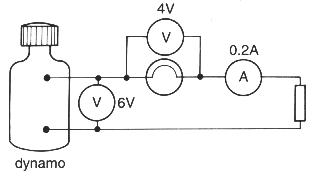
(i) State the formula linking power to current and voltage.
......................................................................................................................................................
.............................................................................................................................................. [1]
(ii) Use this information to calculate the power of the lamp in the circuit.
.............................................................................................................................................. [2]
(iii) The student then connected the dynamo to two identical lamps.
Using ideas about resistance and energy, explain why one lamp was dimmer than the other.
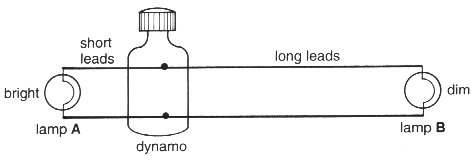
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [2]
6 Prostaglandins are produced when the body is cut, burned or becomes infected. They are released into the blood. They raise the temperature of the affected area and nerve endings are stimulated causing irritation.
(a) Explain why prostaglandins can be described as acting like hormones.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [3]
(b) Aspirin, codeine and paracetamol are all pain relievers.
Aspirin stops production of prostaglandins. It also reduces the blood's ability to form clots.
Codeine blocks the nerve impulses inside the brain and central nervous system. It can cause drowsiness and may be habit forming.
Paracetamol stops the production of prostaglandins in the brain instead of at the site of injury. Too much paracetamol can cause liver damage.
Aspirin is given to people who have suffered a heart attack.
(i) Explain why this might reduce the chances of another heart attack.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [2]
Information on the active ingredients of four brands of pain relievers is shown in the table.
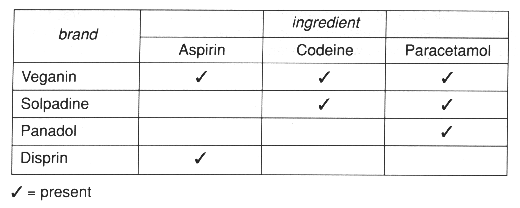
Arthritis is a long term condition which causes pain in joints.
(ii) Use the information to suggest which brand sufferers should take.
Explain your choice.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [2]
7 Grouse are birds that can be infested with parasites which cause bleeding. Infested grouse are more likely to be eaten by foxes which smell the blood and so can find the grouse.
(a) Some grouse bleed a lot more than others when infested by the parasites. Use your knowledge of selective breeding to suggest how you could produce a flock of grouse that are less likely to bleed when infested.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [3]
(b) Gamekeepers want to breed grouse which bleed less. Two parent grouse have the gene combinations (genotypes) BB and Bb, where b is the recessive allele (gene) that causes resistance to bleeding and B is the dominant allele (gene) that allows bleeding.
(i) Fill in the boxes in the table to show the possible genotypes (1, 2, 3 and 4) of the offspring.
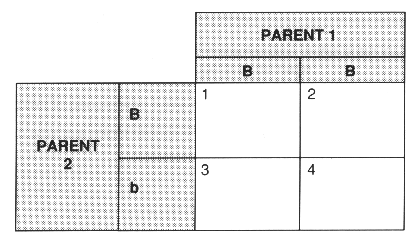
[2]
(ii) For each genotype write down whether the grouse will be resistant or not resistant to bleeding.
genotype 1..................................................................................................................................
genotype 2..................................................................................................................................
genotype 3..................................................................................................................................
genotype 4.......................................................................................................................... [2]
(c) Researchers are hoping to modify grouse DNA using bacteria in order to increase resistance to bleeding.
Some people think that it is wrong to alter the DNA of organisms by genetic engineering. Suggest some arguments which might be used against this type of genetic engineering.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [5]
8 The figure below represents the nitrogen cycle.
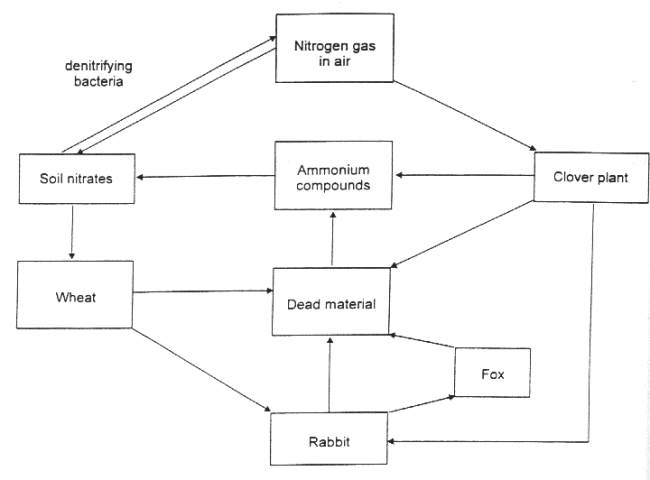
(a) Bacteria play an important part in the nitrogen cycle.
(i) Denitrifying bacteria are anaerobic. Explain why plants growing in waterlogged soil may show signs of nitrogen deficiency.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [3]
(ii) Why might a nitrogen deficient soil limit the growth of the plants?
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [2]
(b) Explain why a good crop of wheat would require the addition of a fertiliser containing nitrogen compounds, while a crop of clover grown in the same field would not need the addition of nitrate fertiliser.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [4]
(c) Why are artificial fertilisers normally applied directly to crops in the spring but manure is added to the soil in the autumn?
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [4]
9 When a new small town was built, two different schemes to supply energy were considered.
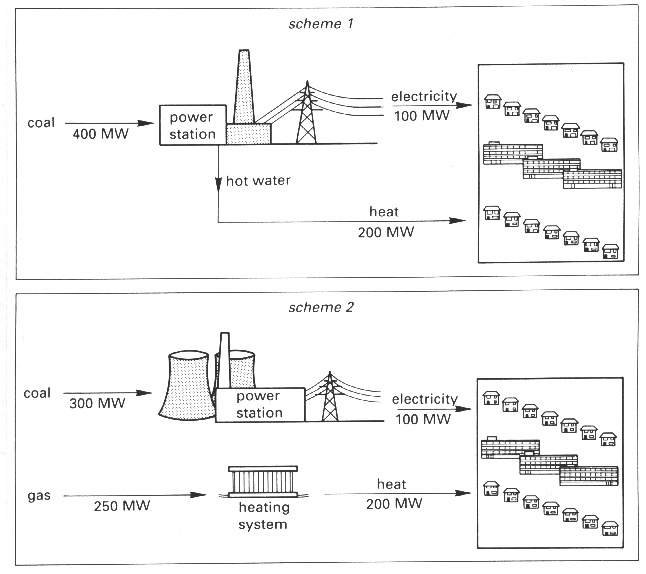
(a) Which fossil fuel provides the energy for heating in:
scheme 1........................................................scheme 2........................................................[1]
(b) Complete the table below.
total power needed total input power in MW
by the town in MWscheme 1 300 scheme 2 300 [1]
(c) The efficiency of a power station is measured using the formula
useful output (in MW)
efficiency = ------------------ x 100
input (in MW)
The answer is in the form of a percentage (%).
Use this formula to calculate the efficiency of the power station in scheme 2. Your answer should be correct to the nearest whole number.
.............................................................................................................................................. [2]
(d) Suggest a reason why power stations are not 100% efficient.
......................................................................................................................................................
.............................................................................................................................................. [1]
10 A manufacturer of small electrically powered pumps sent the following memo to its research department.
MEMO: Please find out how the supply voltage affects
the current passing through our low voltage
pumps.
(a) Draw a circuit diagram to show how the investigation could be carried out.
[5]
The graph shows the results of the investigation.
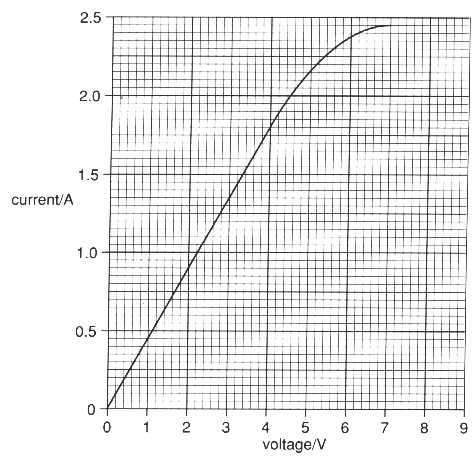
(b) Use the information from the graph to calculate the electrical power used by the pump at its normal operation voltage of 3V.
.............................................................................................................................................. [3]
(c) The research department report includes the statement that:
'above 1.7A the windings of the motor become hot.'
(i) What indication is there on the graph to justify this statement?
.............................................................................................................................................. [1]
(ii) Suggest what changes cause this indication on the graph.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [2]
(d) Apart from the windings becoming hot, suggest other energy losses which might occur when using such a pump.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
............................................................................................................................................... [2]
11 The diagrams show various monomers and the polymers that can be made from them.

(a) (i) Which structural features do all these monomers have which allows them to be polymerised?
.............................................................................................................................................. [1]
(ii) What name is given to all compounds containing this structural feature?
.............................................................................................................................................. [1]
(b) (i) Draw the structural formula for part of the polymer of tetrafluoroethene.
[1]
(ii) What is the chemical name of this polymer whose common name is 'Teflon'?
.............................................................................................................................................. [1]
(c) Explain two environmental problems which are associated with the disposal of plastic objects.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [4]
12 Motor car bodies, and other things made from iron or steel, can be damaged by rusting.
(a) Why is rusting of iron described as a chemical change?
.............................................................................................................................................. [1]
(b) A scientist collected information about the rate at which iron rusts.
Information was taken in places which have different annual rainfalls.
test site A B C D E annual rainfall in mm 425 570 600 900 1000 corrosion rate in units 80 100 110 150 180
Using the grid, plot the values of corrosion rate against annual rainfall and draw a suitable line to complete the graph. The first point has been plotted for you.[3]
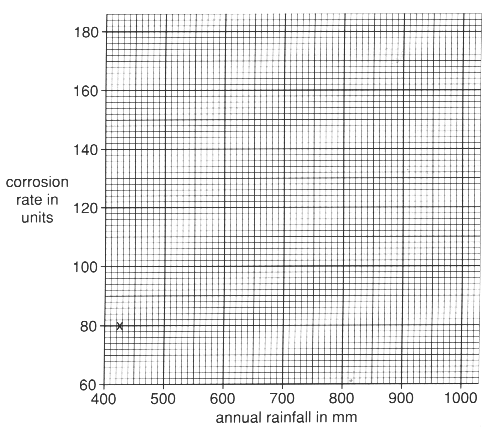
(c) Use the graph to describe how corrosion is linked to annual rainfall.
......................................................................................................................................................
.............................................................................................................................................. [2]
(d) (i) Suggest one other factor which may affect the rate of corrosion.
.............................................................................................................................................. [1]
(ii) Explain how this factor would affect the rate of corrosion.
......................................................................................................................................................
.............................................................................................................................................. [2]
(e) A new paint is said to 'protect against rusting'. Briefly describe an experiment you could do to test this, given some nails and a sample of the paint.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [3]
13 Strontium-90 is a radioactive isotope which was carried by winds when the nuclear power station at Chernobyl caught fire. As a result some sheep still have increased amounts of strontium-90 in their bodies.
Strontium is in Group II of the Periodic Table.
(a) Using the Periodic Table, write down:
(i) the relative atomic mass of strontium.
.............................................................................................................................................. [1]
(ii) the atomic number of strontium.
.............................................................................................................................................. [1]
(b) The most common isotope of strontium is strontium-88. How does an atom of strontium-88 differ from an atom of strontium-90?
......................................................................................................................................................
.............................................................................................................................................. [2]
(c) Milk is an important source of calcium in our diet. Use your knowledge of the Periodic Table to answer the following.
(i) Why are strontium compounds taken into our bodies so easily?
......................................................................................................................................................
.............................................................................................................................................. [1]
(ii) What is the charge on a strontium ion?
.............................................................................................................................................. [2]
14 This question is about a shuttle in orbit.
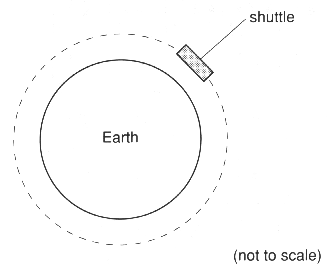
The shuttle and its crew are kept in orbit by the force of gravity between it and the Earth.
(a) Draw an arrow in the shuttle to show the direction of this force. Label it W.[1]
(b) Explain why the crew inside the shuttle appear to be weightless and can float around the cabin.
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
......................................................................................................................................................
.............................................................................................................................................. [4]
(c) The crew switch on the shuttle's rockets for a short while. This increases the speed of the shuttle. Draw the new orbit of the shuttle on the figure above.[1]
The periodic table - click here for a 100k+ image