7Ee/1 Rainbow fizz
In this activity you will be using your senses to make observations.
You will also need to follow instructions carefully.
If you are careful, you should be able to produce a rainbow effect in the tube.
Apparatus
- test tube rack containing four test
tubes and a boiling tube, with chemicals in them
- eye protection
Method
1 The boiling tube contains sodium carbonate. Describe the appearance of the substance in the tube.
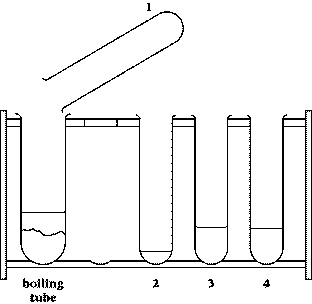
2 Test tube 1 contains water. Add the water to the boiling tube. Feel the outside of the tube. Describe what you see and what you can feel.
3 Shake the tube. Describe what you can see now.
4 Tube 2 contains universal indicator. Smell the tube carefully by wafting the smell towards you. Describe the appearance and the smell of the liquid in the tube.
5 Add the universal indicator to the boiling tube. Observe the tube without shaking. What do you see?
6 Now shake the tube. What do you see now? What type of substance is sodium carbonate?
7 Tubes 3 and 4 contain ethanoic acid. Smell each tube carefully. Describe the appearance and the smell of the liquid in the tubes.
8 Add the contents of tube 3 to the boiling tube. Do not shake the tube. Describe what happens.
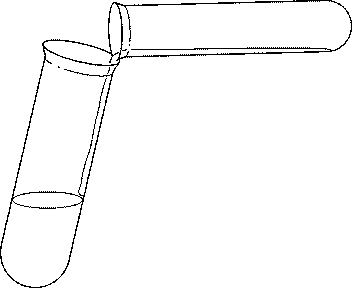
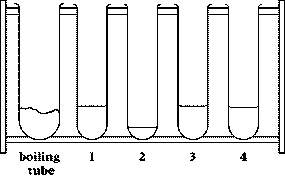
9 Very carefully pour the contents of tube 4 into the boiling tube. You may find it helpful to tilt the boiling tube slightly as shown in the diagram.
Do not shake the tube. Describe what you see.
10 Explain why you get the 'rainbow' effect.
[
observing ]